Trekrezan®
The product with the fastest antiviral effect, which treats ARVI, boosts the immunity, and regenerates energy
Action
Trekrezan® stimulates the natural induction of α- and γ-interferons, providing rapid and long-term antiviral effect1
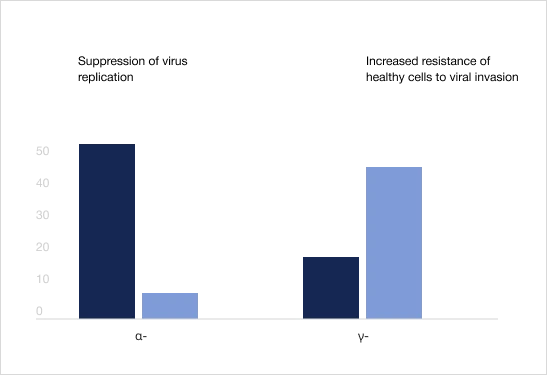
Interferon levels in blood serum after a single dose of the product, IU/mL
Studies
Trekrezan® quickly eliminates the ARVI symptoms2
Results of an observational study of Trekrezan® efficacy and safety in adult patients with ARVI:
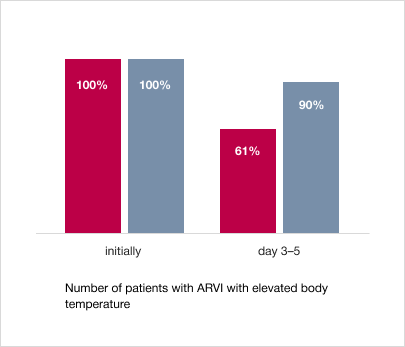
1. Faster normalization of body temperature
In the group of patients taking Trekrezan®, there were significantly fewer patients with elevated body temperature on days 3–5 of therapy
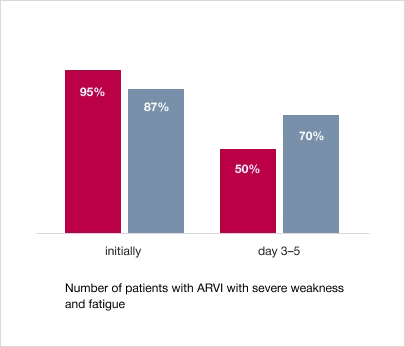
2. Reduction in the severity and duration of the main symptoms of the disease – weakness and fatigue
In the treatment group taking Trekrezan®, on day 3–5 of therapy, there were significantly fewer patients with severe weakness and fatigue
Additional Properties of Trekrezan®1
Anti-toxic
The destruction of virus-infected cells causes an increase in toxic load (temperature, muscle aches, etc.)
Trekrezan® stimulates the activity of phagocytes, which subject toxins to lysis, and also stimulates the activity of liver enzymes.
Anti-asthenic
In the process of viral intoxication and virus control, tissue hypoxia occurs in the cells of the body. Therefore, after the recent illness a patient may feel exhausted for a long time.
Trekrezan® reduces the oxygen demand of cells.
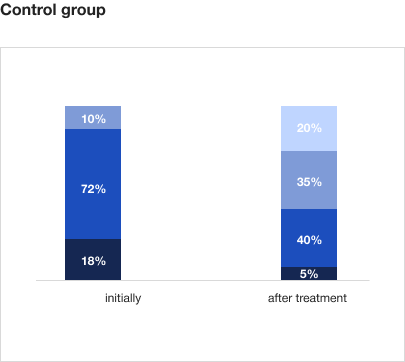
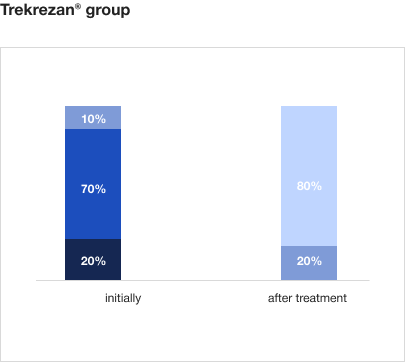
Trekrezan® eliminates the asthenia symptoms in 80% of patients who have recovered from COVID-193
On therapy with Trekrezan®:
- The number of patients with severe asthenia decreases from 20% to 0%, and the number of patients with moderate asthenia decreases from 73% to 0%.
- Emotional well-being and sleep quality improve compared to the control group.
ROUTE OF ADMINISTRATION
For adults and children aged 12 and older
ARVI TREATMENT
thrice a day
per day
ARVI PREVENTION
per day
14 days
IFN inducer drugs IFN inducer drugs are recommended for inclusion in treatment regimens for mild forms of COVID-19*
* Temporary methodological recommendations (TMR) “Prevention, diagnosis, and treatment of novel coronavirus infection (COVID-19)”, Version 12 of the Ministry of Health of the Russian Federation dated 21.09.2021, p. 40
Objective
To evaluate the safety and efficacy of Trekrezan® (200 mg tablets) based on the dynamics of the condition of patients with mild to moderate ARVI.
Inclusion criteria:
- confirmed diagnosis of acute respiratory infection of the upper respiratory tract (J00–J06) of mild to moderate severity with other respiratory manifestations (J10.1; J11.2);
- presence of at least two of the common symptoms of ARVI with other respiratory manifestations (rhinorrhea, stuffy nose, sore throat, cough, sneezing, headache), as well as asthenovegetative syndrome;
- body temperature (axillary) from 37.5°C to 39°C inclusive.
Objective
To study the Trekrezan® efficacy and safety in patients with post-infectious asthenia who have recovered from COVID-19 at the outpatient stage
Treatment group: 40 patients aged 18 and older who took Trekrezan® according to the following regimen: on Day 1 – 3 tablets (600 mg). Next 7 days – 1 tablet (200 mg) per day. The duration of therapy was 8 days (total dose of the product – 2000 mg).
Control group: 40 patients aged 18 and older who did not receive therapy.
Diagnosis:
- the recent COVID-19 within one month that did not require hospitalization.
THERE ARE CONTRAINDICATIONS. IT IS NECESSARY TO CONSULT A HEALTHCARE PROFESSIONAL.
